Abstract
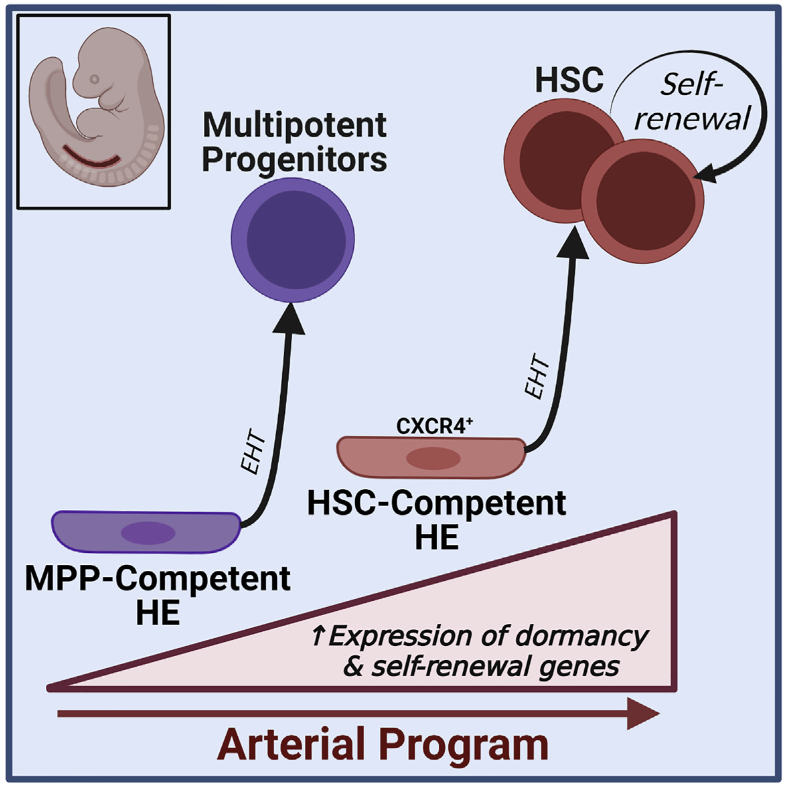
During embryogenesis, waves of hematopoietic progenitors develop from hemogenic endothelium (HE) prior to the emergence of self-renewing hematopoietic stem cells (HSCs). Although previous studies have shown that yolk-sac-derived erythromyeloid progenitors and HSCs emerge from distinct populations of HE, it remains unknown whether the earliest lymphoid-competent progenitors, multipotent progenitors, and HSCs originate from common HE. In this study, we demonstrate by clonal assays and single-cell transcriptomics that rare HE with functional HSC potential in the early murine embryo are distinct from more abundant HE with multilineage hematopoietic potential that fail to generate HSCs. Specifically, HSC-competent HE are characterized by expression of CXCR4 surface marker and by higher expression of genes tied to arterial programs regulating HSC dormancy and self-renewal. Taken together, these findings suggest a revised model of developmental hematopoiesis in which the initial populations of multipotent progenitors and HSCs arise independently from HE with distinct phenotypic and transcriptional properties.