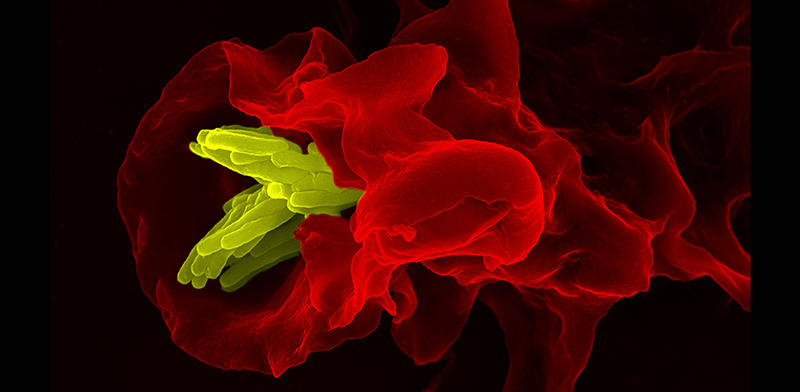
 Macrophage engulfing Tuberculosis bacteria.
Courtesy of Dr. Volker Brinkmann, Max Planck Institute for Infection Biology, Berlin Germany.
Macrophage engulfing Tuberculosis bacteria.
Courtesy of Dr. Volker Brinkmann, Max Planck Institute for Infection Biology, Berlin Germany.
To fight infection, maintain tissue homeostasis, and heal injuries, the cells of the human immune system perform a staggeringly complex spectrum of molecular tasks. Recent studies of differentiation of monocytes into macrophages and dendritic cells have questioned the sharpness of the boundaries between these cell types. The Trapnell laboratory is using macrophages as a model system for investigating cellular specialization. We aim to unveil the molecular regulation of macrophage specialization by using single-cell genomics to survey macrophages from different tissues and polarizing (or repolarizing) them into different states.
Polarization and repolarization in host defense
Upon phagocytosis and presentation of foreign peptides to T cells, macrophages are activated and “polarize” into an transcriptional program that helps clear infection. Recent studies have dramatically expanded the range of known functions performed by highly specialized macrophage subsets. Macrophages can be polarized into the “M1” state, which amplifies local tissue inflammation, as well as several others including an “M2” state, which plays an important role in reducing inflammation and promoting healing. However, whether M1 and M2 macrophages are defined, discrete subtypes as opposed to ends of a continuum of functional states is unclear. The functional diversity of macrophages has challenged the classical view of the monocyte-derived hierarchy. Moreover, how monocytes and their derivatives transition between states is poorly understood. For example, whether the M1 cells are directly “repolarized” to M2 rather than cleared to make way for new monocyte-derived M2 cells is unknown. We aim to investigate the “landscape” of epigenetic, transcriptional, and functional macrophage states and define the signals, receptors, kinases, and transcription factors that determine their “address” within it.
Tissue macrophage specialization
Macrophages are involved in the homeostasis, defense, and repair of nearly every tissue in the body and perform myriad tissue-specific functions. How is regulation of “general” duties of macrophages integrated and balanced with tissue-specific tasks? For example, alveolar macrophages process and clear lipid phagocytosed as part of normal tissue maintenance, primarily through PPARg, a known inhibitor of pro-inflammatory gene expression programming. However, these cells must also defend the lung against inhaled pathogens. Thus, alveolar macrophages would seem to need perform two opposing tasks simultaneously. Are there two specialized subpopulations in lung that coexist? Or is there a mechanism by which a single population can switch between these roles? Does this mechanism require clearance and replacement with fresh monocyte-derived cells? Or might these polarization stages be fully reversible in situ? We are working to understand how macrophages interpret cues from their tissue microenvironment to adopt specialized roles and while maintaining host defense tasks.